Carbon neutrality refers to a state in which the amount of carbon dioxide and other greenhouse gases (GHGs) being emitted is balanced by the same amount being removed (absorbed by forests, thanks to appropriate forest management and forestation), resulting in virtual net-zero emissions. Achieving carbon neutrality is a grave global challenge, with growing implications for ensuring sustainable business. Reducing CO₂ emissions in particular is defined as a key issue by many companies, including Mitsui Kinzoku, which is promoting various business and other activities to contribute to carbon neutrality.
One recent example of such activities is related to a new adsorbing material featuring a porous structure currently under development by the Company. This article provides an introduction to the development of the material and the creation of compact CO₂ separation/recovery devices to implement its application.
Mitsui Kinzoku is striving to reduce CO₂ emissions in various areas, such as the improvement of energy efficiency at facilities, resource conservation through recycling, energy and fuel conversion from fossil to low-carbon fuels, and the use of low-carbon electricity.
Aiming to attain the CO₂ emissions reduction target for fiscal 2030 and achieve carbon neutrality by fiscal 2050, we have formulated transition strategies. A key framework to carry out these strategies consists of the following four approaches: energy/resource saving; energy and fuel conversion; shifting to low-carbon electric power; and carbon offsetting/innovation.
In line with that framework, the Company is promoting technology development to create a CO₂ separation/recovery system and related functions, while proactively working to commercialize development outcomes in order to create new businesses that can contribute to carbon neutrality. Will you explain the key technologies used in the relevant development?
We are developing CO₂ separation/recovery technology by utilizing adsorbent materials and building an overall system to implement the technology.
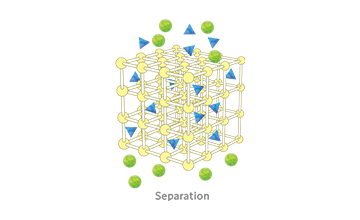
Specifically, our model is centered on a solid CO₂ absorbent used to immobilize a CO₂-absorbing solution within a porous support. It is characterized by a porous support structure composed of three-dimensional network silica frameworks featuring mesopores, with continuous macropores constituting gaps between the frameworks. With this structure, the CO₂-absorbing solution is immobilized in the mesopores within the porous supports, enabling the macropores to act as a gas passageway to increase gaseous diffusion, thus raising the adsorption and desorption processing rate. This structure enables our material to outperform competing products.
By applying this material to the product under development, we are looking to create a compact and highly efficient CO₂ separation/recovery system.
Principle of solid absorption process

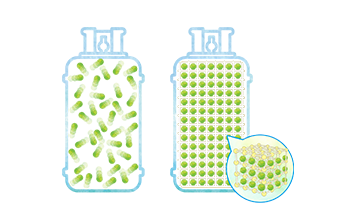
Common-type porous materials are pelletized from powder by extrusion molding. In molded granular materials, interparticle spaces provide the main gas passage, which varies in size.
In contrast, our development product is composed of three-dimensional network silica frameworks with gaps in between that form a continuous macropore through-hole, a structure that helps increase gaseous diffusion. A traffic congestion analogy might help promote understanding. Traffic jams often occur where the road becomes narrower, or the number of lanes decreases. In comparison, our product has a structure that can be likened to a road that extends to infinity with its width unchanged, constituting a property that supports the smooth passage of gas. This gives our development outcome a significant advantage over common-type porous materials.
Results of comparative experiments indicate that a structure with continuous macropores does have an effect on raising the gas diffusion rate, resulting in faster CO₂ adsorption.
In what way can that technology be applied to create a compact device?
One major CO₂ separation/recovery process that has already been in practical use is based on the chemical absorption technique. In this process, CO₂ is recovered from industrial fumes by spraying an amine solution onto the fumes, causing a reaction. This conventional method, however, poses a number of problems, including the need for high-temperature conditions of around 100 to 120°C in order to separate the CO₂ from the amine and then concentrate the separated CO₂, which means a lot of energy is used for heating; large-scale equipment is required to house the structure for spraying the liquid solution; and there is a lower efficiency of CO₂ recovery from industrial fumes with lower levels of CO₂.
Solutions to these issues will be provided by our porous materials development. In addition to the macropores I’ve just described, our materials also have finer nanoscale pores in which CO₂-absorbing solution is immobilized. Exhaust gas injected into the porous material rapidly diffuses through it via the macropores. In the process, it comes into contact with the CO₂-absorbing solution immobilized in the nanopores, producing a highly efficient CO₂ recovery process.
As I understand it, CO₂ adsorbents reach their full capacity in the course of processing, meaning there is a limit on the amount that can be processed. How is this problem solved?
One solution to this problem is to build a process to enable the reuse of the CO₂ adsorbent that has reached its full capacity. For this purpose, you need some way to remove CO₂ from the CO₂-absorbing solution, typically involving a pressure operation. Here, it is crucial to create a fast-alternating adsorption-desorption process in order to increase the performance of the adsorbent, which will help downsize the device.
The method of enabling this process has already been established for practical use. In this method, two or more adsorption towers are prepared within the device, and all towers engage in the same operation by turn, with each following a sequence of procedures to perform the operation. Specifically, they continue the operation until full capacity is reached and then shut the valve to switch to the closed mode, whereupon the vacuum pump works for depressurization to promote CO₂ desorption in order to produce high-purity CO₂. This is one way to achieve the continuous operation of the CO₂ separation and recovery process.
Our plan is to apply the above existing method to our new materials, looking to increase efficiency in CO₂ recovery.
CO₂ separation/recovery process

(Date of interview: December 2, 2024)